- Learning organic chemistry is like learning a new language. With a new language comes a new way of speaking and a new way of writing. In organic chemistry we communicate with lines and letters.
- How to draw bonds
- Bonds are represented by lines. One line is a single bond, two lines is a double bond and three lines is a triple bond
Bond Drawing Single 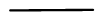
Double 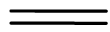
Triple 
- Bonds may be in a variety of different planes
Drawing Representation 
Drawn to represent a bond within same plane as the computer screen or paper 
Drawn to represent a bond coming out of the plane of the computer screen or paper. In essence this bond is pointing toward you. 
Drawn to represent a bond going into the plane of the computer screen or paper. In essence this bond is pointing away from you.
- Bonds are represented by lines. One line is a single bond, two lines is a double bond and three lines is a triple bond
- General rules of drawing in orgo using the “Line-Angle Formula”
- General Rule #1:
- Anytime there is a line drawn, the atom at each end is a carbon unless otherwise indicated.
- The appropriate number of hydrogens are attached to it and implied even though they may not be drawn
- For example, each of these three are equivalent depictions of the same compound:
Condensed Formula Conventional Structure Drawing Line-Angle Formula Drawing CH3CH3 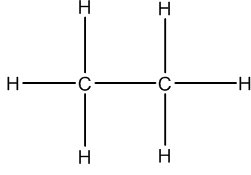

Looking at this Line-Angle Formula drawing we see a line. Each end of the line has a carbon. Since the carbons are connected by a single bond, each carbon has three hydrogens attached to it.
- General Rule #2:
- Each carbon molecule may accommodate four “things” attached to it via a single bond. If there is a double bond that counts as two “things” if there is a triple bond that counts as three “things”
- So what about this molecule:

Letís start by circling all the lines:
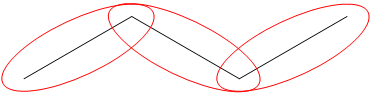
Well, there are three lines, right? Each positioned at an angle to one another to indicate that there is a new carbon at that joint.
So the following two images are equivalent:Conventional Structure Drawing Line-Angle Formula Drawing 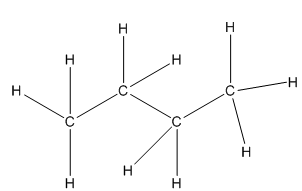

- General Rule #1:
Archive for July, 2012
How To Draw Organic Chemistry Molecules
What’s the difference between General Chemistry and Organic Chemistry?
We get asked this question often. Students who would like to enter health fields will invariably take both of these courses. While General Chemistry is taught in high school, time dedicated to the “organic chemistry” field is usually brief and often not mentioned at all. So why is it that you spend two entire semesters studying both!?
Big Picture:
General Chemistry: the study inorganic compounds (in general those without carbon)
Organic Chemistry: the study of organic compounds (in general those with carbon)
In general, many university level general chemistry courses will cover the following topics over the span of two semesters:
- atomic structure
- the periodic table of elements
- bonding
- study of compounds, moles and molar mass
- stoichiometry
- kinetics, thermodynamics and equilibrium
- ideal gas law
- chemical phases: solid, liquid, gas
- molarity and molality
- redox reactions
- acid – base chemistry
- nuclear chemistry
By studying these areas within general chemistry you learn to understand and appreciate ionic compounds, salts and minerals. You also can gain an appreciation for some of the theory and applications in industry and technology.
So what about organic chemistry? Why spend an entire year learning about carbon?!?
Carbon containing compounds are the gateway to life.
In general, many university level organic chemistry courses will cover the following topics over the span of two semesters:
- atomic structure, nomenclature and resonance
- structural formulas, hybridization and molecular shapes
- stereochemistry
- structure and conformation of alkanes
- alkyl halides
- nucleophilic substitution
- elimination
- alkanes
- alkenes
- alkynes
- alcohols
- gringard reagents
- ethers
- epoxides
- conjugated systems
- aromatic compounds
- aldehydes, ketones and amines
- carboxylic acids and their derivatives
- amino acids, proteins and carbohydrates
- laboratory methods such as spectroscopy
By studying these areas within organic chemistry one can have an appreciation for living organisms and the basis for life. One can also gain an understanding for organic chemistry contributions to health, medicine and pharmacology.
What is a good “leaving group?”
In organic chemistry we use the term leaving group to refer to a portion of a reactant that will depart the rest of the molecule as a result of reacting with another reagent. This occurs often in organic chemistry reactions. Notably in substitution and elimination reactions.
For example in the following SN2 reaction:
CH3CH2Br + ¯OH/DMF → CH3CH2OH
The leaving group is the Br. This is easy to notice because it has “left” the main reactant: CH3CH2Br Now OH is in the place of the Br hence this is a substitution reaction.
Common questions and answers:
#1
So what makes a good leaving group “good”?
Answer: weak bases make good leaving groups
#2
What are some examples of good leaving groups?
Answer:
-OTS (tosylate)
-I
-Br
-Cl
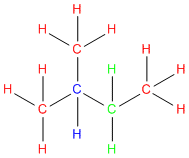
What are primary, secondary and tertiary carbons?
Q&A from our students:
Question: What are primary, secondary and tertiary carbons? I know that sounds like a basic questions, but were just beginning to learn about alkanes and stuff and I don’t get it. Thanks.
Answer: It is a great questions and we’re am happy you asked because there are a lot of students who are confused about this subject. Let’s try to clear the air. Because here at StudyOrgo.com – we love to clear the air and make things easy!
We use the terms primary, secondary, and tertiary to refer to the substitution level that a given carbon has in a molecule. In other words, these terms are used to describe how many other carbons a given carbon is attached to.
So to figure out the substitution level of any given carbon, follow these three easy steps:
Step #1: Pick a carbon
Step #2: Count how many carbons are directly attached to it. Other elements such as hydrogen, nitrogen, oxygen etc. don’t count.
Step #3: Give it a label:
- Primary = a carbon attached to only ONE other carbon
- Secondary = a carbon attached to only TWO other carbons
- Tertiary = a carbon attached to THREE other carbons
In the below example each carbon is color coded using the labels in step #3 above.
Go ahead give it a try!
OK- now bear in mind that hydrogens attached to a given carbon ALSO take on the labels as described in step #3 above.
So we can apply the same principle to the hydrogens:
- Primary = a hydrogen on a carbon attached to only ONE other carbon
- Secondary = a hydrogen on a carbon attached to only TWO other carbons
- Tertiary = a hydrogen on a carbon attached to THREE other carbons
For more on mastering alkanes and reactions, use coupon code “acespring” to save 10% off the highest pass rate organic chemistry program.
You may submit a question to our experts by filling out the form HERE.
You’re question may be answered in an upcoming blog posting!