Deciphering 1H-NMR Spectra
One of the most important concepts taught in organic chemistry is the method for determining the chemical structure of newly synthesized or unknown compounds. In this article, we will summarize the concept of proton NMR, the most common NMR information acquired by organic chemists.
While proton NMR is used every day in the real world by organic chemists, it is also tested every day in the real world by organic chemistry professors. That is why we are bringing you this exclusive StudyOrgo.com simplified review on NMR.
Before you read on, if you like what you see, but are looking for explanations on different organic chemistry topics and reactions, check out how our signature online organic chemistry program works to help you earn higher grades.
Now if you are ready to read on about NMR- here ya go:
Placing an unknown sample in a strong magnetic field allow 1H nuclei (99.98% abundance) to “resonate”, which is when their nuclear spins flip at a unique electromagnetic (EM) frequency (Hertz, Hz). The instrument detects this and plots it on a graph in units of ppm. This value is relative to an internal standard, tetramethylsilane (TMS) which is arbitrarily set at 0 ppm. The signals originating from your unknown sample are recorded as a net difference from the TMS reference. The ranges for common functional groups are shown in the graph below.
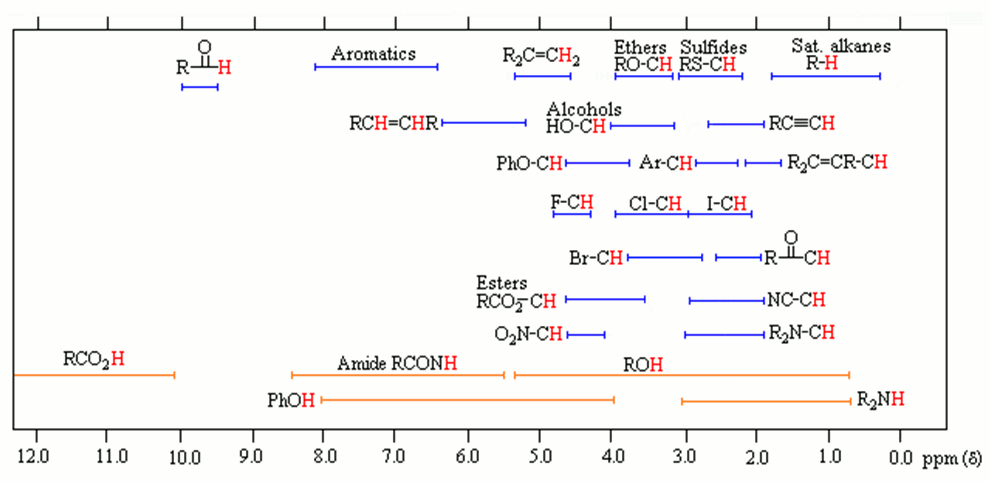
Notice that protons (in red) neighboring or attached to electronegative elements (N,O,S,Cl,Br,I) have a large chemical shift (d). This is colloquially referred to as “downfield” on the spectrograph. This is due to the deshielding effect, which is simply that the electron cloud around protons near electronegative or electron-withdrawing groups are smaller, so less EM radiation is required to resonate the nucleus. Conversely, when the proton is connected to carbons, the chemical shift is <2 ppm. This is because these proton nuclei are “shielded” from the magnetic field and higher energy is required for them to resonate and referred to as “upfield” on the spectrograph. Once a NMR spectrograph is recorded, 4 pieces of information can be determined from the data as long as the chemical formula of the compound is known.
To illustrate the points, we will consider the following 1H-NMR spectrum of the C5H10O.
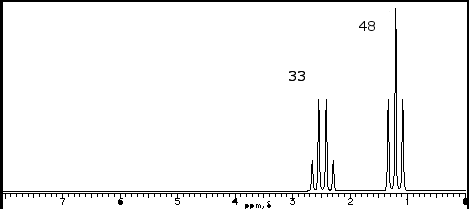
- Signal Count – Number of unique hydrogens
This is the easiest to interpret. The number of peaks correspond to the number of unique, or chemically indistinguishable, hydrogen nuclei. There are two peaks on the graph, therefore of the 10 hydrogens in the molecule, there are two types.
- Chemical Shift – Identity of neighbors
The two peaks on the spectrum are located at (d)2.42 and (d)1.07. Remembering that the chemical formula includes 5 carbons and one oxygen, it is clear there is a good deal of symmetry to the molecule. Furthermore, the only proton near an oxygen that exhibits a chemical shift ~ 2.5 with is neighboring a carbonyl group HCRC=O. So far, we know our compound is symmetric and has a carbonyl group, therefore only 4 carbons can have carbons.
- Integration – Number of Equivalent Hydrogens
Integration is the calculation of the area beneath the peaks. This information is determined by the computer software and would not be required for you to determine from visual inspection. But if it is provided, it will instantly determine the number of “equivalent” (or chemically indistinguishable) hydrogens in your graph. Looking at our spectra, we have integration of 33 + 48 = 81 cart units in total. The integration fraction can be multiplied by the total number of hydrogens from the chemical formula to determine number of equivalent hydrogens. For instance, at (d)2.42 [33/81 = 0.40 * 10 total hydrogens = 4 hydrogens at (d)2.42]. Therefore, we know that the protons near the carbonyl total 4. Given the symmetry from the low peak count, we know these hydrogens must be two CH2 groups. The same for the peak at (d)1.07 [48/81 = 0.60 * 10 total hydrogens = hydrogens at (d)1.07]. Since only 2 carbons are left, we must conclude these are terminal CH3 groups.
- Signal Splitting – Number of Non-equivalent Hydrogen Neighbors
Signal splitting occurs from a phenomenon of coupling, which occurs when NON-equivalent neighboring protons interfere with the resonance of a proton nuclei. The degree of splitting occurs in an N+1 rule. For instance, at d1.07 we have a triplet. This indicates that there are 2 protons neighboring the CH3 group that are different. This would have to be the protons from the CH2 group at (d)2.42. Similarly, the peak at (d)2.42 as a quartet, indicating that 3 protons are neighboring the CH2 group that are different. This would have to be the protons from the CH3 group.
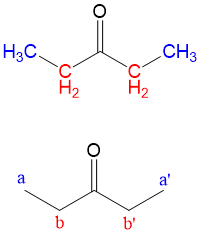
And voila! The chemical structure from the spectrum must be 3-propanone. This is an example of the clear-cut explanations you will receive with your membership with StudyOrgo. With practice and help from StudyOrgo, you’ll be solving your organic reaction problems in no time!
Want to try it about before you purchase? No problem- check out our sample reaction flashcard page.
Ready to sign up? Sign up here.


