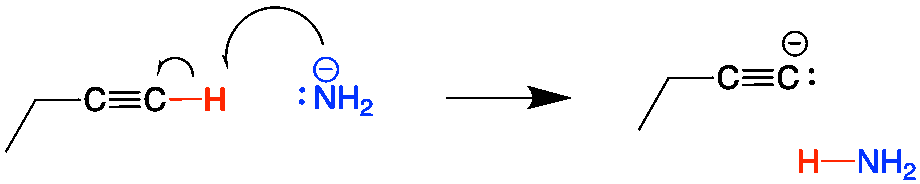Many students taking Orgo 2 have commented there are a few types of reactions the professors save to the end of the semester and cover quickly and “gloss” over or sometimes skip all together in the interest of time. However, you will be responsible for all of the reactions necessary for multi-step synthesis (starting product known to get to unknown final product) and retrosynthesis (product known to get to unknown starting material) reactions. We at StudyOrgo don’t want you to get stuck on trying to cram for exams by studying reactions that were poorly covered in your class. In this article, we focus on how to make new C-C bonds using alkenes.
Alkynide Anion Formation
First, the terminal alkyne must be deprotonated with a strong base, such as sodium amide (NaNH2). This proton can be removed because terminal alkyne hydrogens are more “acidic” than alkene or alkane hydrogens. This forms the alkynide anion, which is a great nuleophile.
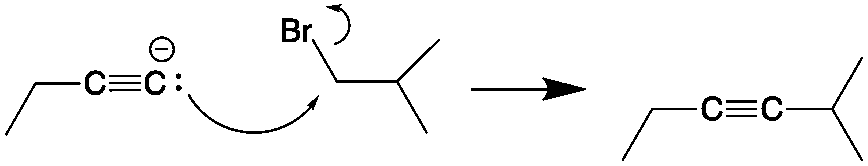
Next, the a alkynide anion must attack a primary or secondary carbon center to perform an Sn2 type reaction. This displaces the leaving group and forms the new C-C triple bond, which has now become an internal alkyne.
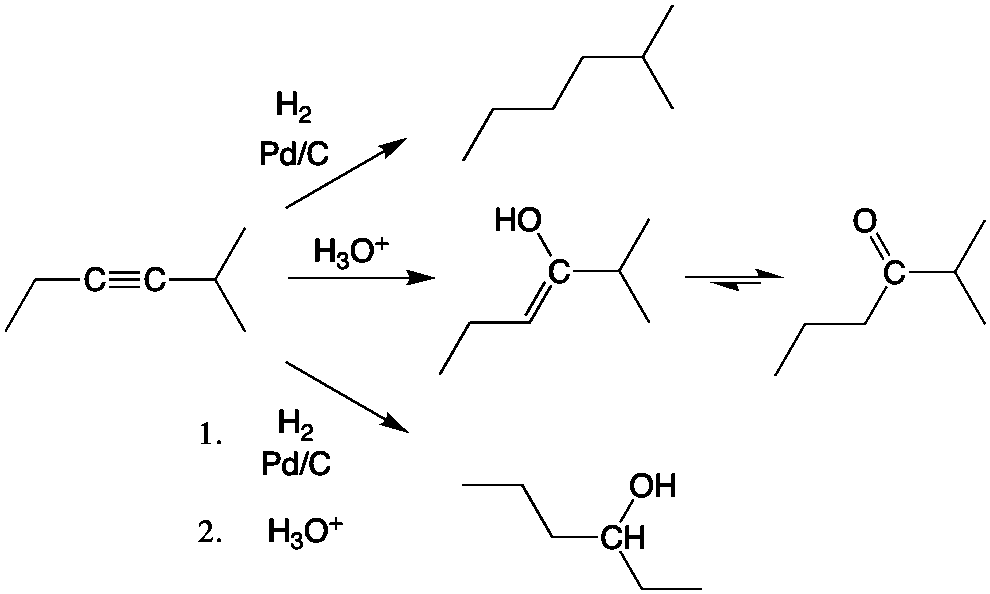
Once you have the the new alkyne, you can choose to reduce the alkyne to an alkene or alkane, or you can transform the alkyne into many other functional groups, including a ketone or an alcohol by hydration.
Remember, alkynes are a great tool to build large carbon skeletons using simple haloalkanes. Remember this reagent for the exam! We here at StudyOrgo have devoted countless hours to preparing complex reaction mechanisms in simple and easy-to-understand manner to help you maximize your studying. Sign up with StudyOrgo for detailed explanations of epoxide reaction mechanisms and other essential Orgo 2 reactions today!