One question that comes up in organic chemistry often is “what is an enol or an enolate and how is it formed?” These types of concepts are frequently covered quickly in class or not at all, but are very important for future reaction mechanisms. We at Study Orgo have the combined experience of over 15 years of tutoring and teaching organic chemistry concepts to struggling students. We have developed clear descriptions of reaction mechanisms and organic chemistry concepts to aid students in their studies. Sign up today for access to over 180 reactions mechanisms and reviews!
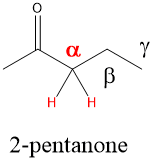
The alpha carbon of a carbonyl, which is present in carboxylic acids, esters, ketones and aldehydes, are acidic which means the proton can be removed using a base. In neutral or acidic conditions, this means the lone pairs on the C=O position can act as a weak nucleophile.
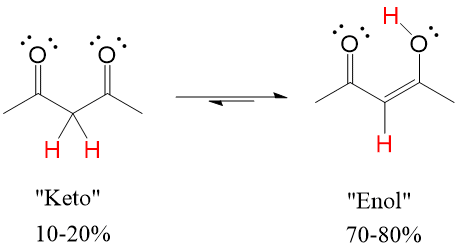
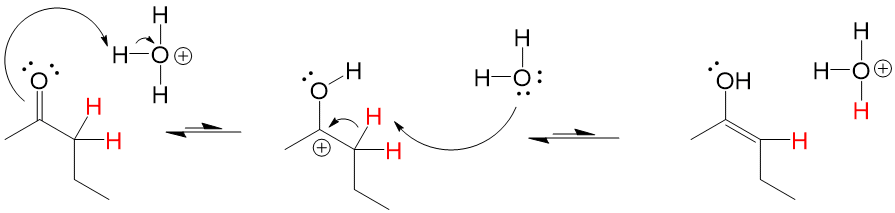
If the carbonyl oxygen can attack the alpha carbon C-H bond, it will abstract the hydrogen and perform a Keto-Enol tautomerization reaction that will lead to the resonance version of the carbonyl, which is the Enol (alkENE + alcohOL)
Enols – rearranging the pi bond and atoms of a carbonyl compound to an Enol
Catalyist: Acidic or Neutral Conditions to stabilize OH formation
Enols tautomers are generally unstable, preferring the “Keto” version 90-99% of the time versus the “Enol” version. However, a catalytic amount of presence is sometimes enough to drive reactions forward if the mechanism requires the enol tautomer of the compound.
However, in some cases such as a beta diketone, shown below, the combined dipoles of two carbonyls makes the alpha carbon very acidic, meaning enol formation is very favorable. In this case, it is 70-90% enol in solution.
Enolates – Deprotonating the alpha carbon and tautomerizing to the oxyanion
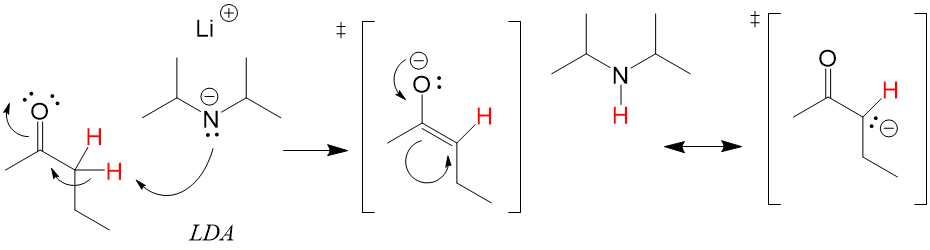
Catalyist: Strong Base to deprotonate the alpha carbon.
Like an Sn2 mechanism, a strong enough base will react with the acidic proton on the alpha carbon and deprotonate. The electron density between the C-H bond will shift to make a new C=C bond, while the C=O electrons will be placed on the oxygen, creating and alkENE + alcohOL anion “ATE”) with a strong base to produce a stable carbanion. The stability is due to the tautomerized structure that can be produced by placing the negative charge on the oxygen.
Enolates are generally forward reactions depending on the strength of the base. How strong the base required depends on the pKa of the alpha C-H bond. In the case of ketones, a strong base like LDA is required. However, for beta dikeontes, a mild base like NaOH is enough to generate the enolate.
Formation of Enols and Enolates are an important source of carbon nucleophiles to make new C-C bonds in future reactions.
Sign up with StudyOrgo today to get access over 180 chemical to reactions with detailed reaction mechanisms, explanations, tips and tricks to each mechanism.