Many students taking Orgo 1 have commented there are a few types of reactions the professors save to the end of the semester and cover quickly and “gloss” over or sometimes skip all together in the interest of time. However, in Orgo 2, you will be responsible for all of the reactions necessary for multi-step synthesis (starting product known to get to unknown final product) and retro-synthesis (product known to get to unknown starting material) reactions. We at StudyOrgo don’t want you to get stuck on trying to cram for exams by studying reactions that were poorly covered in your class.
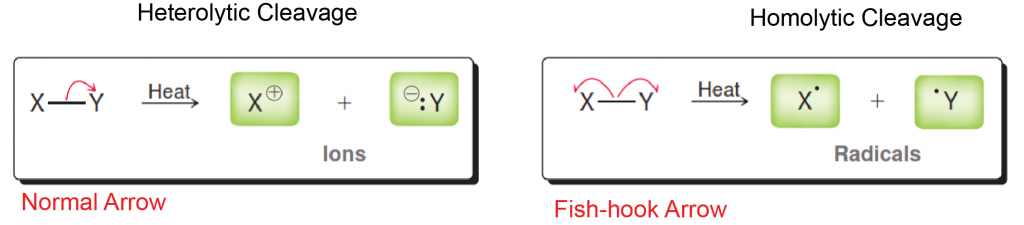
In this article we will review the steps to radical ion formation used in a few reaction including free radical halogenation of alkanes. To begin, the three steps to radical reactions are 1) Initiation, 2) Propagation and finally 3) Termination. The formation of radicals always occurs in the Propagation step of the reaction. Energy has to be supplied to a molecule to induce a reaction known as homolytic cleavage; the breaking of a bond where both atoms receive 1 electron. Most reactions occur by heterolytic cleavage, which means 2 electrons that formed the bond being broke are given to one atom (negative) while the other atom loses them (positive).
There are two methods for initiating radicals, either heat (symbolized as delta) or light (symbolized as hv). To show the homolytic cleavage during initiation, the convention is to draw a fishhook arrow (one sided barb to the arrow) to each atom receiving one of the electrons. In Orgo 1, you mechanisms will be graded on the quality of your fish-hook arrow and what bond the electrons came from to what atoms they are going towards, so be very clear!!!
Below is an example of heterolytic vs homolytic cleavage and how to draw the arrows correctly!
There are two other steps to free radical halogenation that occur.
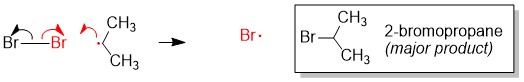
Once the radical is created, it will attack alkane bonds (C-H) of substrate molecules to create H-X and a new radical alkane. This step is referred to as propagation, since the radical is transferred from one molecule to the other essentially. To complete halogenation of the alkane, the radical alkane will attack the abundant halogen (e.g. Cl2, Br2) to form a new C-X bond and generate another radical halogen, just like from the initiation step.
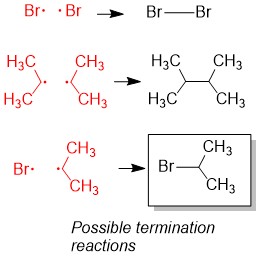
The final step will be termination, where one radical attacks another and now a new bond is formed and no radical product is made.
These concepts are really important to understanding the more complex topics to come. With a membership to StudyOrgo, you will get even more tips and tricks on organic chemistry topics and detailed mechanisms with explanations. Today’s blog is a preview of the detailed topics and materials available. Check out a membership to StudyOrgo.com and sign up today!