Hydrogen bonding is important for describing the driving force of reactions in organic chemistry and a very important concept for explaining the biochemistry of catalytic reactions that occur using protein as enzymes in biological systems. In this post, we will discuss the rules and examples of hydrogen bond formation. We at StudyOrgo have extensive experience instructing principles and reaction mechanisms frequently covered in Organic Chemistry. Sign up today for clear, detailed explanations of over 180 Orgo Chem reactions and reviews on conceptual topics!
Physical properties of molecules such as boiling and melting point, solubility and reactivity, are affected by the functional groups that make up the molecule. This can be explained by analyzing the type of intermolecular forces that are experienced between molecules. Because these forces are not covalent, intermolecular forces are determined by the intensity of electrostatic forces which is what makes up each type of intermolecular force. As a review, the types of intermolecular forces are;
- Van der Waals (London dispersion forces) – Weak, temporary dipole formed between hydrophobic C-H and C-C bonds.
- Dipole-Dipole Interactions: – Strong, permanent dipole moments formed between atoms of functional groups containing bonds such as C=O, C=N, C-O, C-N, N-H and O-H bonds. The delta(-) side of one dipole is attracted to the delta(+) side of another molecule, forming a non-covalent electrostatic attraction.
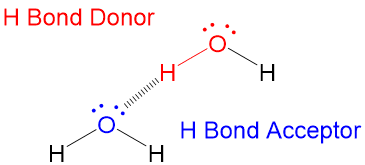
- Hydrogen Bonding: Sharing sharing of a hydrogen atom covalently attached to an electronegative element (typically O-H and N-H groups) between a lone pair of electrons on another electronegative element.
Two terms about hydrogen bonding that are key are;
- The electronegative atom with the lone pair electrons is called the Hydrogen Bond Acceptor
- The electronegative atom bonded to the hydrogen is called the Hydrogen Bond Donor
- The Hydrogen Bond Donor must be aligned 180 degrees to the Hydrogen Bond Donor!
The more intermolecular forces the molecule has, the more energy will be required to disrupt these bonds when melting or boiling compounds, thus raising the observed temperatures from expected relative to their mass. In addition, hydrogen bonds require polar bonds in the molecule and H-Bond Donor proton involved is protic (a donatable hydrogen atom). These are two terms that you will learn in the Sn1 mechanism.
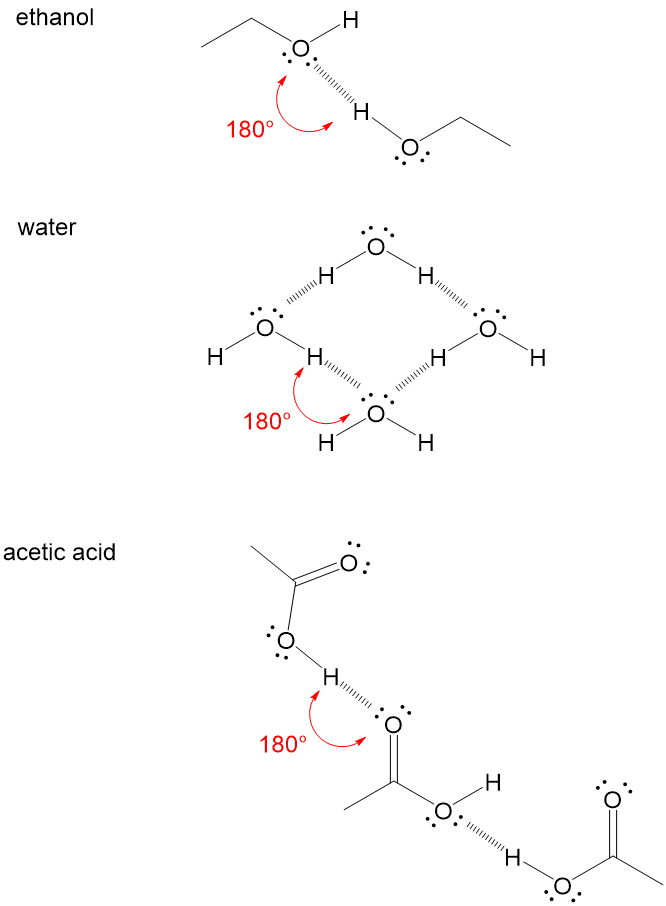
Let’s look at ethanol as an example. The hydrogen bonding occurs between the proton of one alcohol group and the oxygen lone pair electrons on another alcohol group. This is a strong intermolecular force that holds the molecule in a complex 3D shape and makes it easier in reactions to attack the carbon connected to the O-H bond due to inductive effects, or the pulling of electrons away from the carbon. Water is an extreme example, where all the atoms in the molecule participate in hydrogen bonding. The oxygen lone pairs will accept a hydrogen from a neighboring molecule O-H. Finally, acetic acid is another example. Remember, that the H-Bond Acceptor can be any lone pairs, including those of C=O bonds.
These concepts are really important to understanding the more complex topics to come. With a membership to StudyOrgo, you will get even more tips and tricks on organic chemistry topics and detailed mechanisms with explanations. Today’s blog is a preview of the detailed topics and materials available.