- Learning organic chemistry is like learning a new language. With a new language comes a new way of speaking and a new way of writing. In organic chemistry we communicate with lines and letters.
- How to draw bonds
- Bonds are represented by lines. One line is a single bond, two lines is a double bond and three lines is a triple bond
Bond Drawing Single 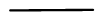
Double 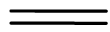
Triple 
- Bonds may be in a variety of different planes
Drawing Representation 
Drawn to represent a bond within same plane as the computer screen or paper 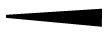
Drawn to represent a bond coming out of the plane of the computer screen or paper. In essence this bond is pointing toward you. 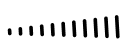
Drawn to represent a bond going into the plane of the computer screen or paper. In essence this bond is pointing away from you.
- Bonds are represented by lines. One line is a single bond, two lines is a double bond and three lines is a triple bond
- General rules of drawing in orgo using the “Line-Angle Formula”
- General Rule #1:
- Anytime there is a line drawn, the atom at each end is a carbon unless otherwise indicated.
- The appropriate number of hydrogens are attached to it and implied even though they may not be drawn
- For example, each of these three are equivalent depictions of the same compound:
Condensed Formula Conventional Structure Drawing Line-Angle Formula Drawing CH3CH3 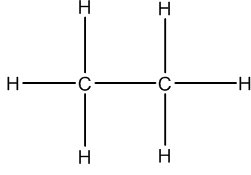

Looking at this Line-Angle Formula drawing we see a line. Each end of the line has a carbon. Since the carbons are connected by a single bond, each carbon has three hydrogens attached to it.
- General Rule #2:
- Each carbon molecule may accommodate four “things” attached to it via a single bond. If there is a double bond that counts as two “things” if there is a triple bond that counts as three “things”
- So what about this molecule:

Letís start by circling all the lines:
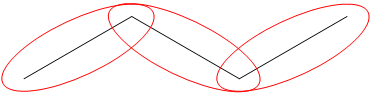
Well, there are three lines, right? Each positioned at an angle to one another to indicate that there is a new carbon at that joint.
So the following two images are equivalent:Conventional Structure Drawing Line-Angle Formula Drawing 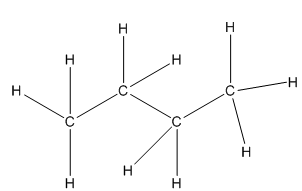

- General Rule #1:
For practice on this subject try out our exercise set at:


