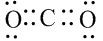Question from one of our Twitter followers:
“How to calculate the no.of non-bonding e- around “a” atom.& how to make sure whether the organic substance is major or minor”
Answer from one of our StudyOrgo.com Experts:
I think what you are asking about two things: how to draw a Lewis structure and how to determine which resonance structures are major and which are minor contributors.
First I will address the Lewis structure:
A Lewis structure is a way to draw out electrons and bonding by using dots. In a Lewis structure some of the electrons are “bonding electrons” and others are “non-bonding electrons” known as “lone pairs.” Here is a simple step by step process using CO2 as an example:
- Step 1: Determine the number of valence electrons an atom has to participate in bonding.
- Definition: Valence electrons are the electrons in the outermost shell of an atom. Some valence electrons participate in bonding to another atom. Others, known as lone pairs, do not.
- The number of valence electrons that participate in bonding for popular atoms are as follows:
- C = 4
- N = 3
- O = 2
- F = 1
- H = 1
- For example: In CO2 we have C (draw 4 dots) and O (draw 2 dots per atom)
- Step 2: Place a dot around that atom for each valence electron that participate in bonding
- For example:
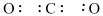
- For example:
- Step 3: Determine the lone pairs for a given atom:
- Remember: Lone pairs are a subset of the valence electrons that do not participate in bonding to another atom
- Lone pairs for popular atoms are as follows:
- C = 0
- N = 1 pair = 2 electrons
- O = 2 pairs = 4 electrons
- F = 3 pairs = 6 electrons
- H = 0
- For example, in CO2 we have C (no lone pairs) and O (two lone pairs per atom)
- Step 4: Place the lone pairs around the atom using a pair of dots to depict each lone pair.
- For example:
- For example:
- Step 5: Wherever there is an atom bonding to another atom there are two dots between them, one from each atom. You may decide to convert this pair of electrons into a line to denote a bond.
- For example:
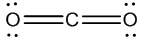
- For example:
- How do I figure out an atom’s formal charge?
- Step 1: Count the atom’s lone pair electrons
- Step 2: Count one from each pair of electrons that particular atom is using to bond to another atom
- Step 3: Add the number you get from Step 1 to Step 2
- Step 4: The formal charge is whatever you need to do to the number you got from step 3 to get to the atom’s group number on the periodic table
- For example: Let’s use an O atom in CO2 as an example
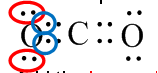
Add the lone pair electrons (4) to one from each pair of bonding electrons (2) = 6
Since oxygen is in group 6 in the periodic table the formal charge is 0 (zero). (You do not need to do anything to the number you get from step 3 to get to the atom’s group number on the periodic table)
Next I will address the Resonance Structures:
- A drawn structure with a double bond on its own does not completely represent the structure of a given molecule
- There can be more than one possible structure for the same molecule!
- The actual structure is the average of all of the resonance structures
- Why resonance?
- Resonance spreads the charge over two atoms which makes the structure more stable
- How do I figure out resonance problems? Follow these simple rules:
- Rule #1: Try moving around electrons.
- When moving electrons use an arrow to demonstrate where the electrons are going.
- Electrons can be moved around in one of two ways:
- Move double bond electrons
- Move lone pair electrons
- Rule #2: The number of unpaired electrons must remain the same
- Rule #3: Figure out which of your drawings represent the major and minor structures
- Major resonance = the resonance contributors that are more stable as they have the least energy. Low energy structures satisfy as many of the following as possible:
- There are as many octets as possible
- There are as many bonds as possible
- There are as few lone pairs as possible
- Any negative charges are placed on the most electronegative atoms
- Most electronegative F > O > Cl > N > C least electronegative
- There is the least separation of charge amongst the structures
- Minor resonance = the resonance contributors that are less stable as they have the most energy. High energy structures do not satisfy as many of the above guidelines
- Major resonance = the resonance contributors that are more stable as they have the least energy. Low energy structures satisfy as many of the following as possible:
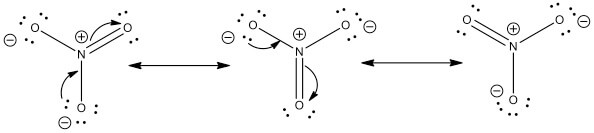
- Example: NO3-
- In the following example NO3- is drawn out showing three different resonance structures. Please remember that while electrons are moving around no atoms are moving.
- The arrows show the movement of the electrons to show how to arrive at the next structure moving from the left to the right of the screen.
- Since all three structures satisfy the same guidelines to the same extent as outlined above, all three are equal contributors. However this is often not the case and will be seen in the next exercise set.