Gearing up for Organic Chemistry this Fall semester is a smart and efficient way for reaching and maintaining a great grade in this class. Most students find the pace of this class very accelerated compared to other courses. This is because while there is a lot of information to learn, it also builds on previous concepts from general chemistry, a course most students want to forget!
But don’t worry! StudyOrgo has you covered. Our Editors have spent years tutoring and teaching Organic Chemistry to students and we have seen all of the pitfalls common to the first few weeks of the semester. Our online platform allows members to learn organic chemistry concepts and mechanisms quickly and the material presented in an easy-to-follow format. Follow along with us and sign up with StudyOrgo today to help prepare you for all of your Organic Chemistry questions.
One of the concepts you will need to have mastered before you begin the class is Hybridized Orbital Theory.
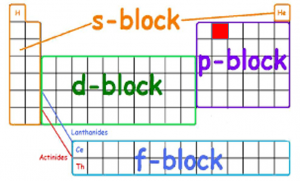
Lets look at carbon, the atom this class is about. Recall that it has atomic number 6, which means it has 6 electrons. Remember that the rows, or periods, of the periodic table reflect the outer electron orbital. In the second period, carbon has 4 electrons in its outer orbital. These are the electrons that are available for bonding. The carbon atom wants to eventually achieve 8 electrons to fulfill the Octet Rule, so it needs to make 4 covalent bonds. Below is how the electrons are filled in their orbitals. Recall that the columns, or groups, of the periodic table reflect the type of electron orbitals present in the atom. The red block is the location of carbon, which indicates that carbon has 2 s-orbital and 2 p-orbital electrons.
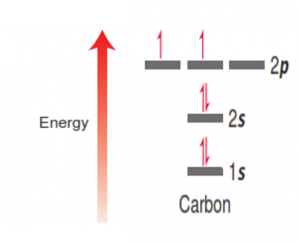
These electrons are arranged in an energy diagram according to their energy, shown below
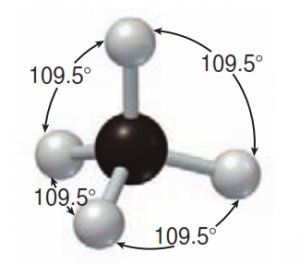
But it we consider the molecular shape of methane (CH4), we will observe it as tetrahedral, where all 4 C-H bonds appear to be equal, so how do the electron energies become equal?
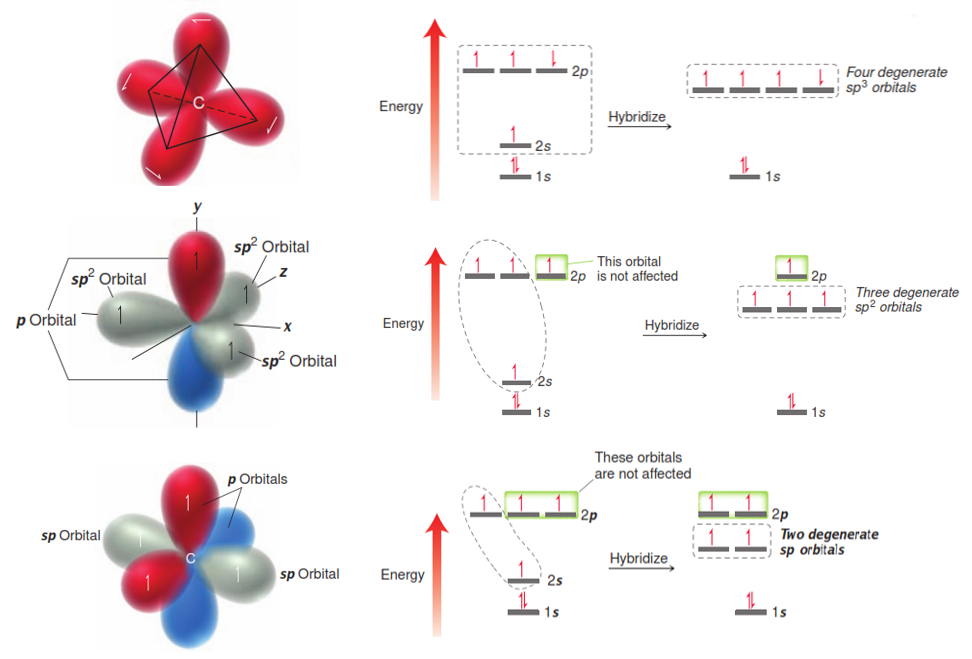
We explain this observation by assuming that the s-orbital and p-orbital electron energies, while different merge into an sp3 hybridized orbital. In this model, the 2 s-orbial and 2 p-orbital electrons merge into something that resembles a dumbbell. Since all of the orbitals have the same shape and energy, the C-H bonds of methane can be equal and form the observed tetrahedral orientation. There are three types of hybridized orbitals for carbon;
- sp3 – which has 4 single (sigma) bonds. The molecular geometry is tetrahedral.
- sp2 – which has 3 single (sigma) bonds and 1 double (1 pi) bond. The molecular geometry is trigonal planar.
- sp – which has 2 single (sigma) bonds and 1 triple (2 pi) bonds. The molecular geometry is linear.
With a good study plan and help from StudyOrgo, you’ll have no trouble passing your organic chemistry quizzes and exams this semester. Sign up today for more comprehensive and clear-cut explanations on all of your organic chemistry topics today!