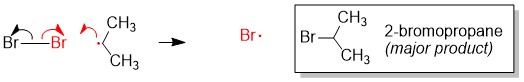
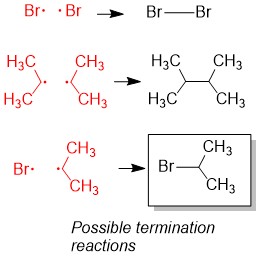
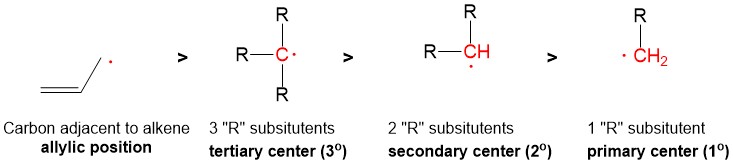
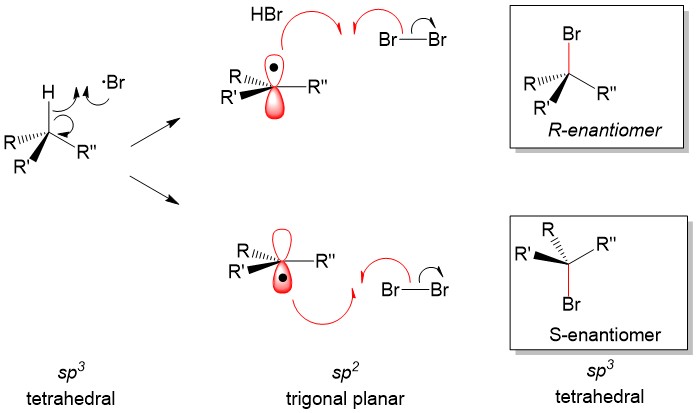
One of the questions we are repeatedly asked at StudyOrgo is “how do I to get ahead in organic chemistry this fall semester?” Many of you have heard that organic chemistry is a brutal class that does little but to depress your GPA. While it is true that this course is challenging, we here at StudyOrgo are devoted to helping you get the “A” you deserve!
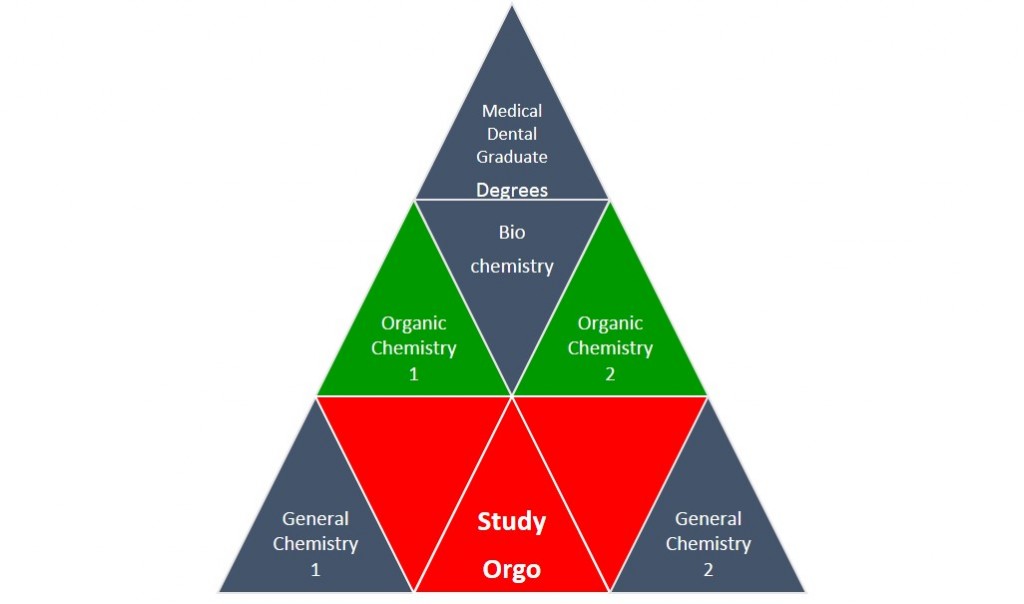
Organic chemistry gets a bad name because it assumes that you are experts with regards to all of the general chemistry from freshman year, and you are now responsible to know it! As an analogy, think of your chemistry courses as a pyramid to reaching your degree goals. Organic chemistry is directly placed in the middle of the pyramid, it will be very important not only for the MCAT or DAT exams, but also for future advanced courses. Organic chemistry is supported by General Chemistry, which is why you took it last year. Fortunately, StudyOrgo is placed in the center of your pyramid base and we are here to help all of your organic chemistry questions. Our simple and clear-cut explanations of reaction mechanisms and concepts will easily help you with anything you might struggle with this semester. Here are a few tips on how to prepare today for the course this Fall.
- Open your text book –Read the title and abstract on the first page of each chapter and check out the number of pages. It will give you a very quick idea of what you will be learning about in each chapter and how much material you will be covering.
- Look at a syllabus – Remember, your syllabus is an official contract between you and the professor. They must disclose what you are required to learn and how you will be graded. Professors can remove requirements but cannot easily add them. Use this to your advantage! Highlight the contents or reactions of the book that will be required and use this to focus your attention on while studying over the semester.
- Schedule your studying! – Now that you know where the book is and a rough idea of what you are responsible for learning from the syllabus, take a calendar and divide the time you have to each test by the number of chapters. Schedule 2-3 hours a week to study and DON’T SKIP OR RESCHEDULE. Think of it as a doctor or dentist appointment – you just have to do it! Also, if you plan your studying ahead, you will be less likely to schedule something that gets in the way because you will already have penciled it in! Use your Smartphone calendar to send you alerts and reminders for your studying appointment.
- Read ahead – If you have time this summer, read at least two chapters to get yourself ahead of the class. Don’t try to understand everything, just pay attention to the words used and the ideas. This will allow you to pay more attention and ask questions about the details in class instead of scrambling to write down notes and drawings.
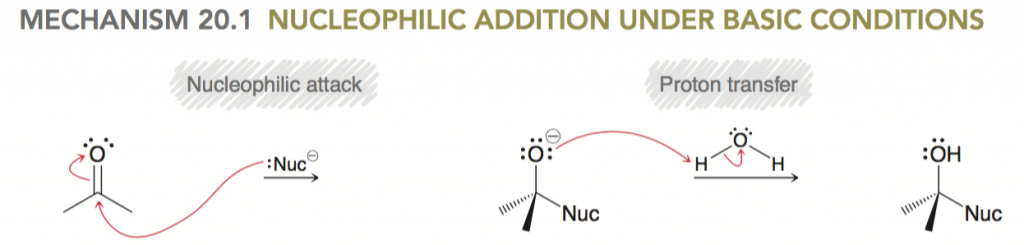
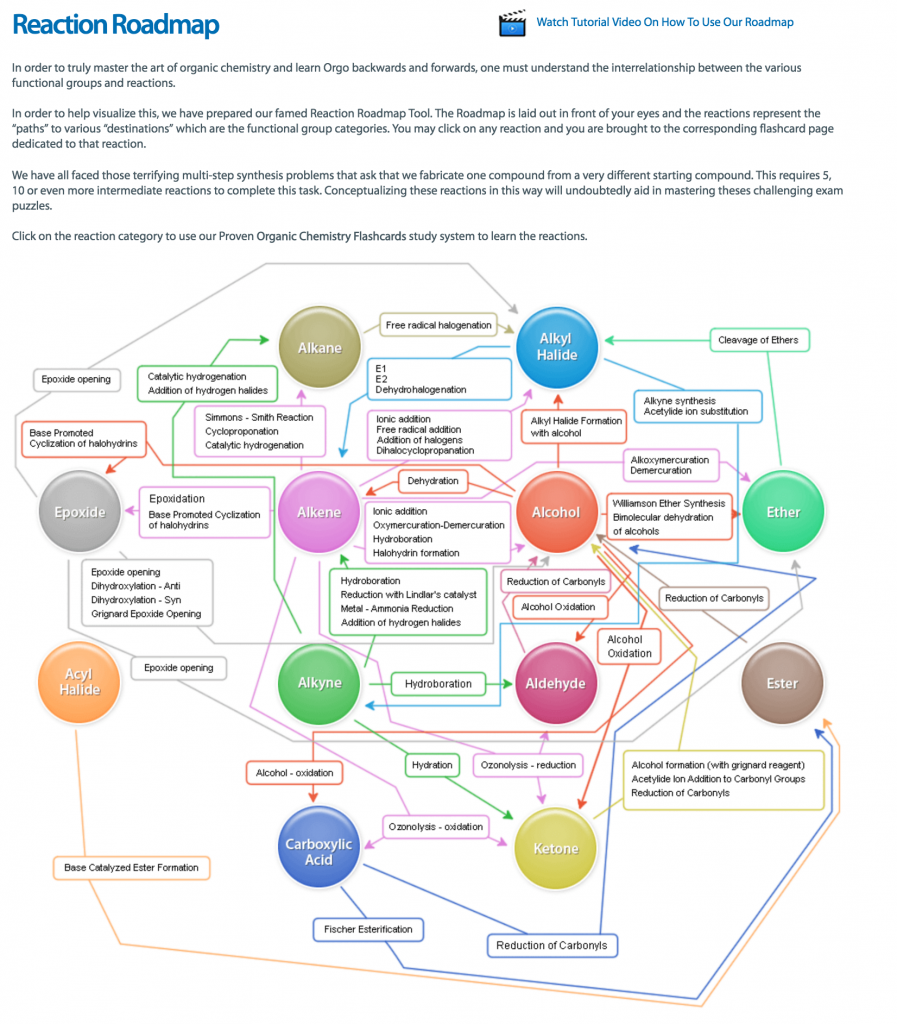
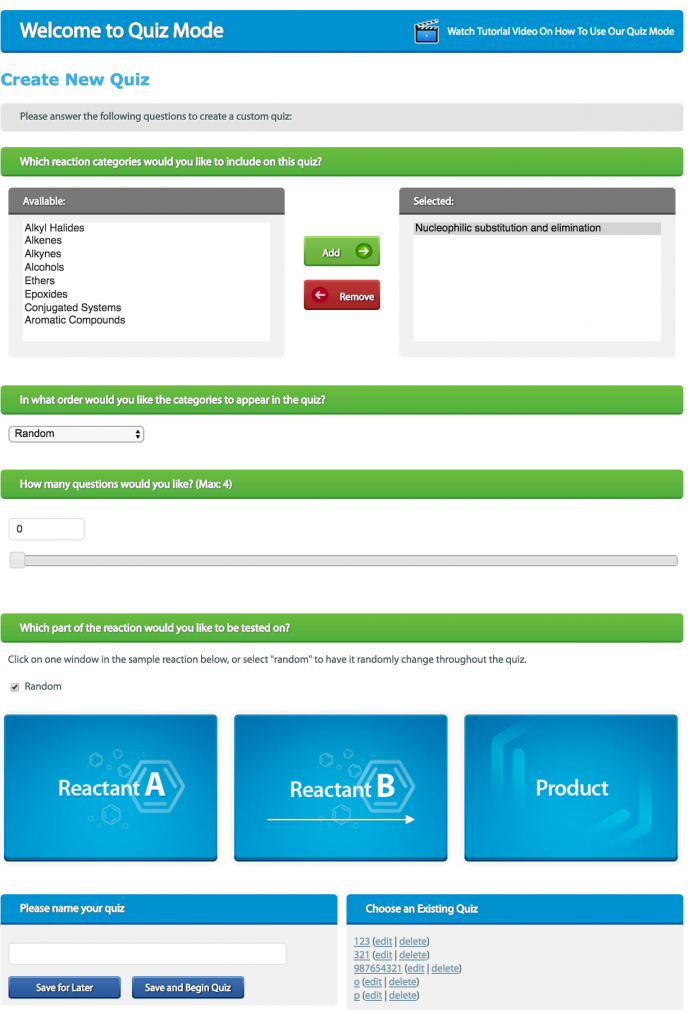
- Sign up with StudyOrgo – The Editors at StudyOrgo have spent numerous hours reviewing and preparing the material in the most crystal-clear and “get-to-the-point” manner as possible. We consult students and ask for their opinion on whether they understand the material as presented. We provide quick descriptions and in-depth mechanism explanations. Many of our reaction have multiple examples, so you can learn and then quiz yourself in our website! For the student on-the-go, we have also developed a mobile app (iOS and Android) provides all the functionality of the website! All of these benefits are included in your StudyOrgo membership!
With a little time management and help from StudyOrgo, you will have no trouble getting an “A” in Organic Chemistry this year!