One of the most important skills to master in organic chemistry is the ability to assign stereochemistry. We at StudyOrgo have devised clear cut explanations of these difficult concepts for students to maximize their time studying and learn difficult concepts quickly and easily. Sign up with StudyOrgo.com today for all of your organic chemistry studying needs!
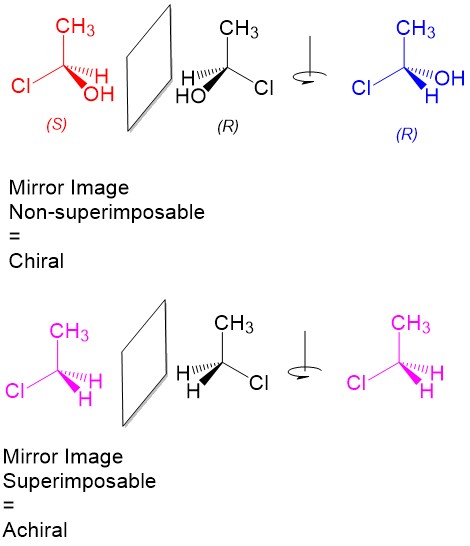
Chirality is an important aspect of life. This is so because many of the basic molecules used in living cells, in particular amino acids that form enzymes, are also chiral. Chirality imparts asymmetry on our molecules, allowing them the ability to recognize “handedness” and further add to the complexity and specificity of reactions. As organic chemists, we must pay constant attention to the chirality of molecules both before and after reactions, less the compounds lose their biological or chemical activity.
Chirality is defined as any object in which the mirror images are not superimposable. A good example is your hands; they are mirror images but not superimposable. Translating this to organic molecules, a stereocenter is a carbon center with 4 unique substituents that are arranged such that the mirror image is not superimposable. Thus, they “look” like to different molecules although they have the same substituents. If we alter the arrangement of the substituents, we can always come up with 2 arrangements for each substituent, R or S configuration. Thus, each stereocenter must have 2 stereoisomers.
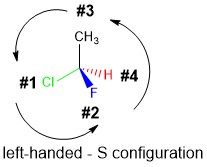
In order to determine whether the sterecenter is the the R or S configuration, there are a series of steps to follow.
- Identify the stereocenter as 4 unique substituents attached to the chiral center
- Assign priority based on atom atomic number, highest (1) to lowest (4) weight.
- If two atoms are same, move to next bond to find first point of difference
- Rotate the molecule so that Priority 4 atom is in the hashed wedge position.
- Determine the Priority sequence 1-2-3 rotates to the left (S) or the right (R).
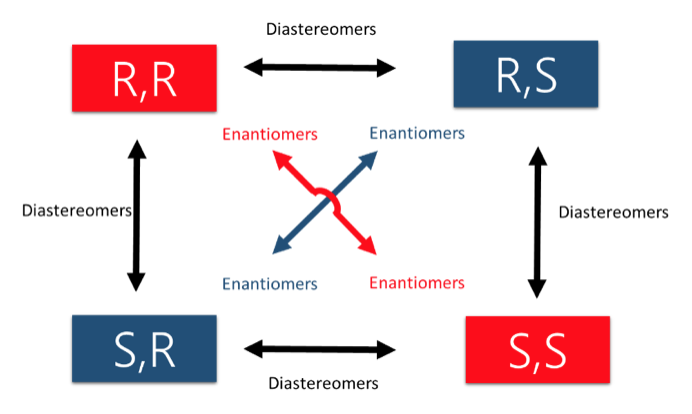
Lastly, an important concept to keep in mind is that as molecules become more complex, they also can acquire more stereocenters. Keeping in mind that each stereocenter can produce 2 stereoisomers, we describe possible stereoisomerism using the 2n rule. Let’s examine a molecule with 2 stereocenters, following the 2n rule that gives us 22=4 stereocenters. The possible combinations are listed below.
We now introduce the last concept to stereochemistry which is the difference between enantiomers and diastereomers. Enantiomers are molecules with exactly opposite stereoisomers. For example, the enantiomer of the molecule with stereochemistry R,R would be S,S. The relationship between molecule R,R and R,S is what is described as diastereomers, which differ in some but not all stereocenters.
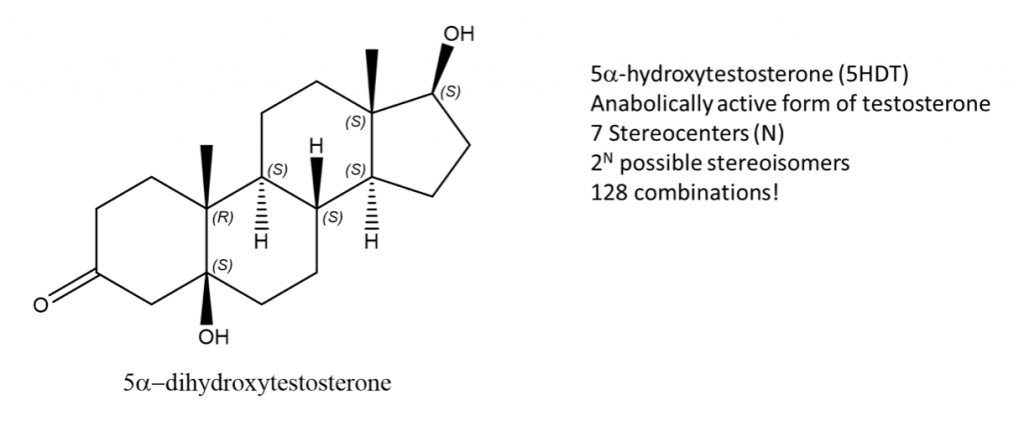
Let’s consider the biologically active form of testosterone, 5-DHT which is shown below. We indicate that it has 7 stereocenters in the molecule. Applying the 2n rule, we calculate 128 possible stereoisomer combinations. That concludes that while testosterone has 1 enantiomer, it has 126 diastereomers and remember…only 5-DHT works on our bodies!


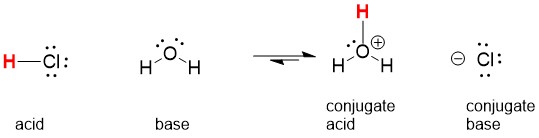
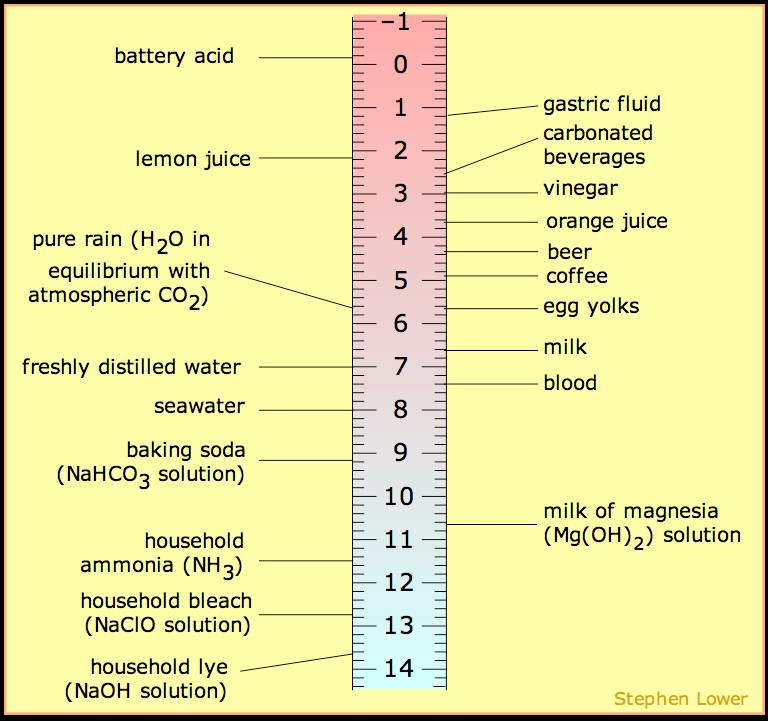
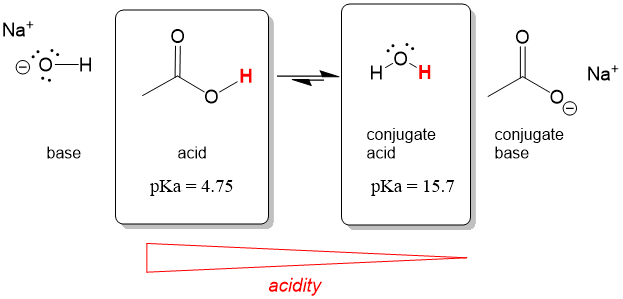
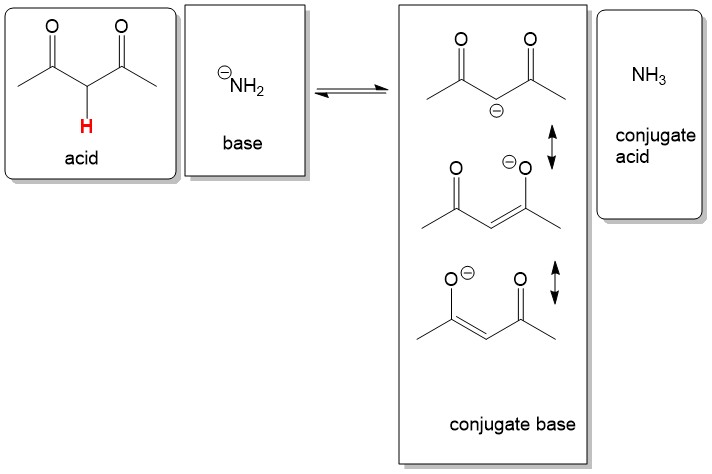
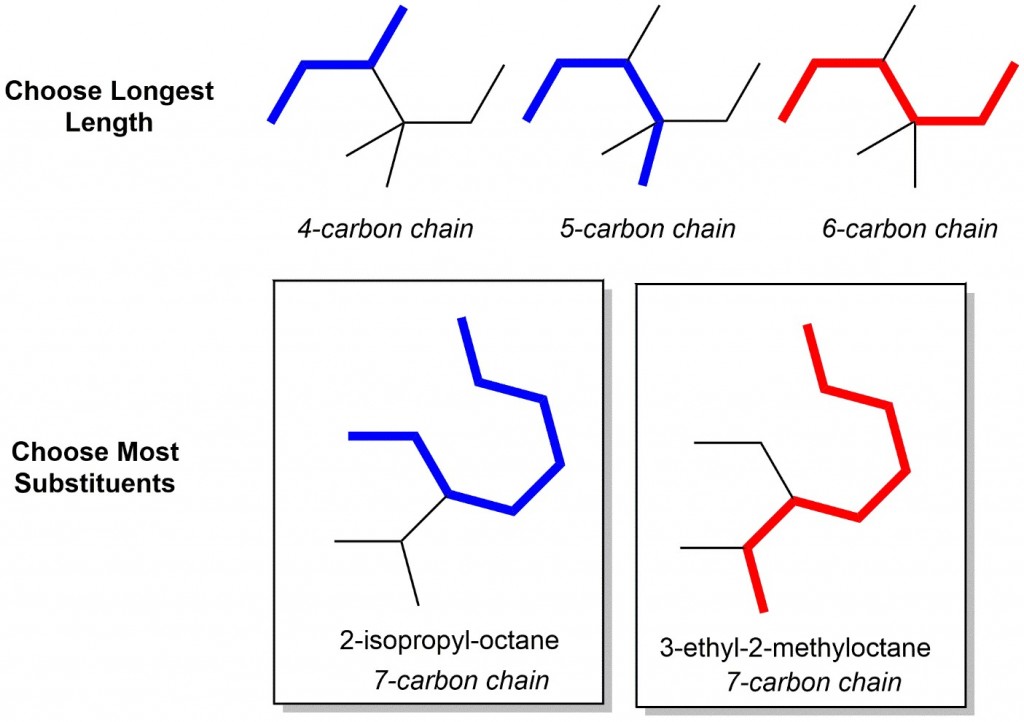
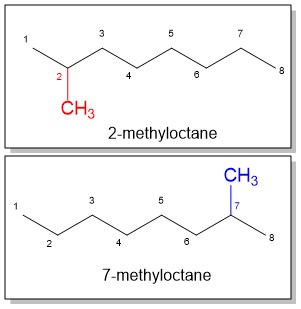
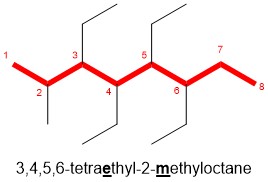

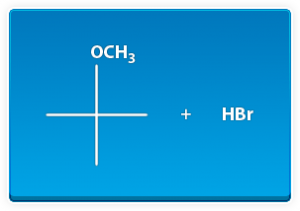 The best way to stay ahead of the semester is to divide your chapters into blocks that you will study. Most professors will have a fairly clear outline of what chapters from your book will be covered before each exam. Your goal here should be to divide your time (starting right now!) until the exam into blocks to study. Some chapters, like substitution (e.g. SN2) and elimination (e.g. E2), are longer than others because these reactions are the foundations for future reactions you will learn later in the course. So, spend a lot of time mastering these important topics. Finished a chapter early? Move ahead and don’t wait until you receive the lecture to start studying. Many people find studying before the lecture help them to really understand what the professor is saying and will give you an opportunity to ask questions right away.
The best way to stay ahead of the semester is to divide your chapters into blocks that you will study. Most professors will have a fairly clear outline of what chapters from your book will be covered before each exam. Your goal here should be to divide your time (starting right now!) until the exam into blocks to study. Some chapters, like substitution (e.g. SN2) and elimination (e.g. E2), are longer than others because these reactions are the foundations for future reactions you will learn later in the course. So, spend a lot of time mastering these important topics. Finished a chapter early? Move ahead and don’t wait until you receive the lecture to start studying. Many people find studying before the lecture help them to really understand what the professor is saying and will give you an opportunity to ask questions right away.