Professors often begin to expect students to perform synthesis (reactant to product) or retrosynthesis (product back to available reactants) on the exam. This can be one of the most intimidating exercises because the products and reactant can look so different from each other it would seem at first glance its not possible to figure it out. But here at StudyOrgo, we work to bring you clear explanations and clear-cut explanations to tackle many organic chemistry reactions and synthesis questions you will see in Orgo1 and Orgo2.
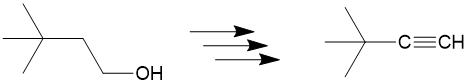
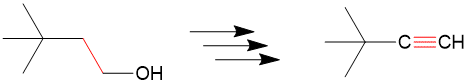
Let’s take a look at the retrosynthesis of 3,3-dimethylbutyne from 3,3 dmethylbutanol. At first you may see the reagent has an alcohol group but maybe you wonder “how am I going to get to an alkyne, we haven’t even seen alkynes yet!”
The first step is to see which bonds have changed between the products and reactants and we can see the bond colored in red. Since only one bond changed, this is relatively “easy” example.
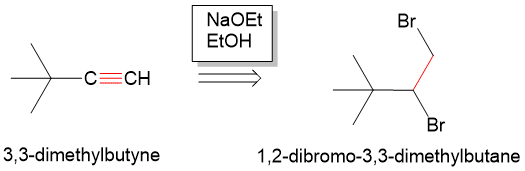
Starting from an alkyne, remember that each double bond represents the presence of a pi bond that can be formed from an elimination reaction. So, think “what can we eliminate that would have resulted in pi bond formation?” Remembering E2 reaction mechanisms, any neighboring anti-periplanar proton and Br (leaving group) can be eliminate with a strong base to form a new pi bond. In the case of the alkyne, we would need two leaving groups and two rounds of elimination to form an alkyne. Thus, if we form two Br bonds to each of the carbons, we can then form the alkyne from 1,2-dibromo-3,3dimethylbutane. In the forward reaction, we can do this with a strong base such as sodium ethoxide (NaOEt) in ethanol to form the alkyne.
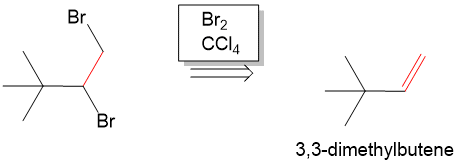
Next, we have to form the haloalkane. To do this, we would have to carry out an addition reaction. Seeing that we need a Br on each carbon, it is not too difficult to realize that if we added Br2 across an alkene, then we can then from the haloalkane from 3,3 dmethylbutene. In the forward reaction , we can do this by reacting the alkene with Br2 in chloroform to form the haloalkane.
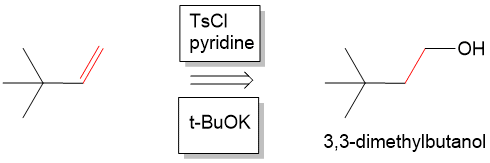
Next, we have to form the alkene. At this point we are looking for a way to come back to the starting material, which is 3,3 dmethylbutanol. We could think of forming an alkene from 3,3 dmethylbutanol by performing another E2 elimination (or dehydration) to remove the alcohol and anti-periplanar proton which would form 3,3-dimethylbutene. In the forward reaction, we can do this by activating the alcohol with tosyl chlroide to make a very good leaving group and then reacting with a strong base such as potassium tert-butoxide (t-BuOK) to form the alkene.
And there you have it! A three step synthesis from the alkyne product from a starting alcohol using reactions that are typically covered in the first few weeks of class. Practice is the only way to learn these tricks and the more reactions you know, the easier it will be to find shortcuts to faster synthesis schemes and solving more complicated examples which will surely be on your final exam. Sign up with StudyOrgo.com today to master over 180 organic chemistry reactions and learn the mechanisms fast!