“How can I tell if a hydrogen is a wedge or a dash in a chair skeleton?”
Here at StudyOrgo, we frequently get questions about topics in organic chemistry that are usually quickly covered, poorly described or expected that you know from previous courses. These concepts are really important to understanding the more complex topics to come. In this article, we will cover the concepts of stereochemistry descriptions using bold and wedged bonds. This is just a preview of the detailed topics and materials available with your membership to StudyOrgo.com. Sign up today!
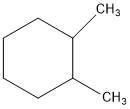
The first thing we have to do is determine is how you want to orient you molecule. Let’s take (1R, 2R) 1,2-dimethylcyclohexane for example. If we orient the molecule to have the methyl groups on the right side, we see that we have two stereocenters available. But the current drawing doesn’t indicate the stereochemistry yet. That’s what the bold and hashed bonds will indicate.
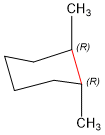
Next, we have to visualize the cyclohexane ring in the chair conformation. Remember, that the skeleton image shown above is more conveniently drawn, but loses the 3rd dimension information, so you have to put it back in the chair to determine which should be bolded and which should be wedge.
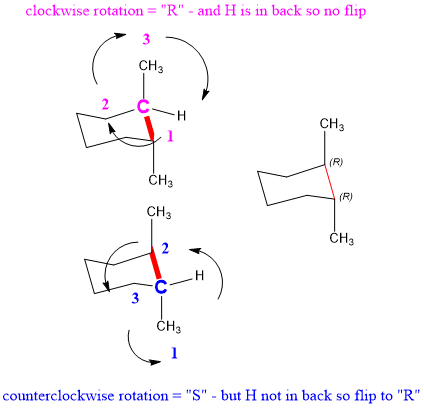
Next, we have to confirm that that the stereochemistry is correct. To do this, you need to practice selecting most important substituents and rotating to assign stereochemistry. Follow along with the examples below, using the blue and pink carbons shown.
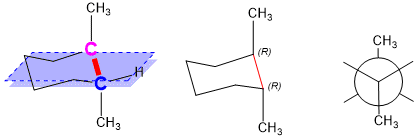
At this point, you should be able to see how the hashed and bolded bonds are now appropriately drawn. The pink stereocenter will be bolded, suggesting it is above the plane of the ring and the blue stereocenter will be hashed, suggesting it is below the plane. Drawing the Newman Projection down the red bond shows that the methyl groups are “anti” to each other, making this a stable conformation.