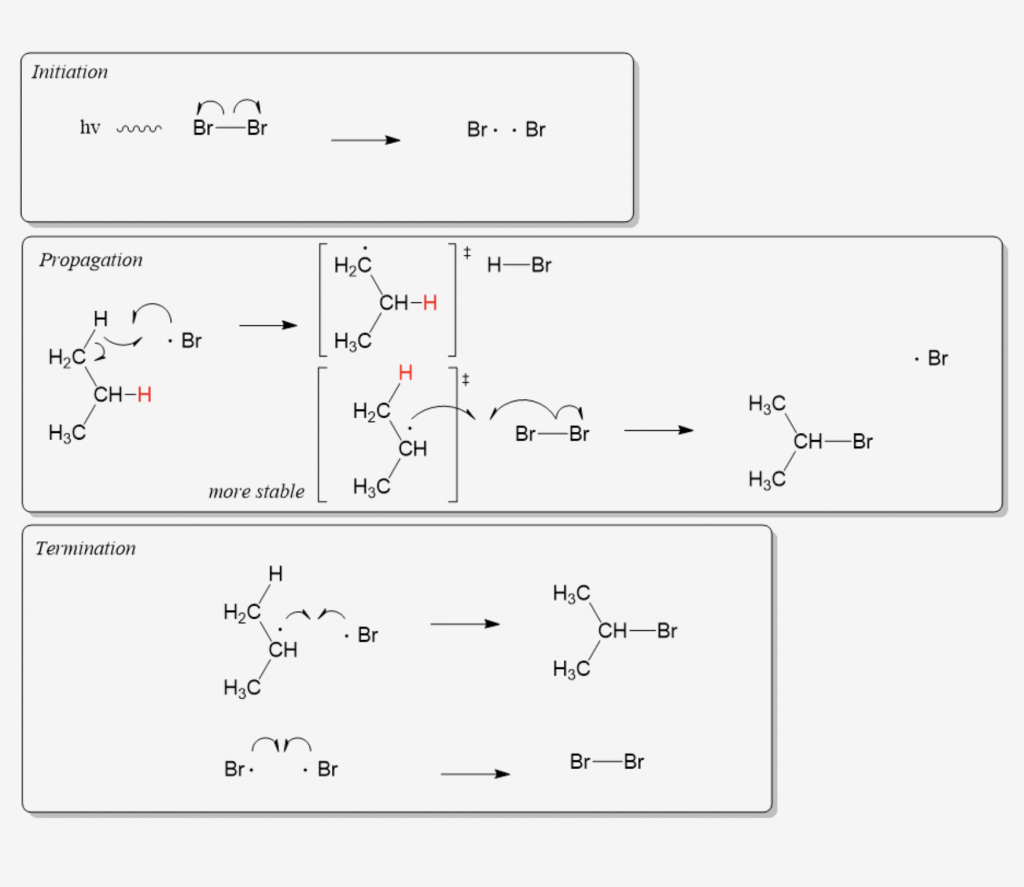
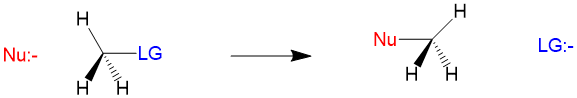
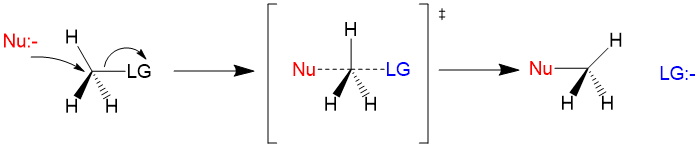
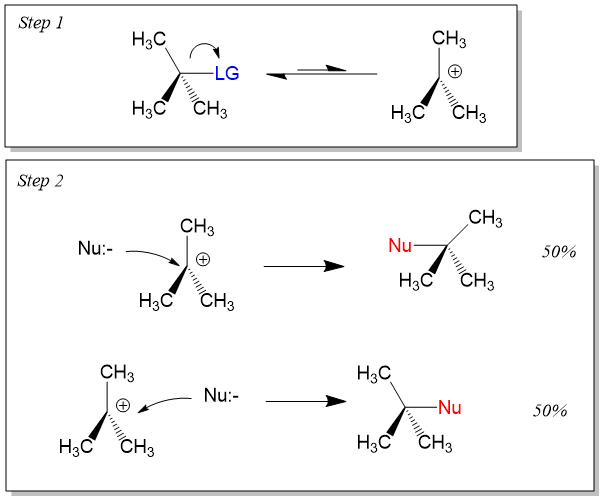
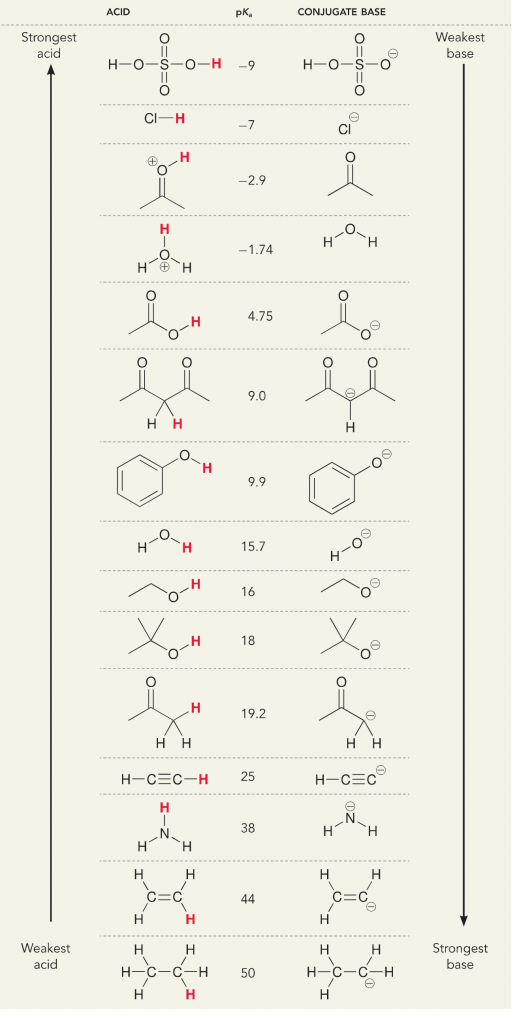
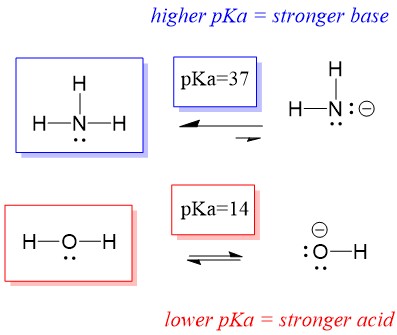
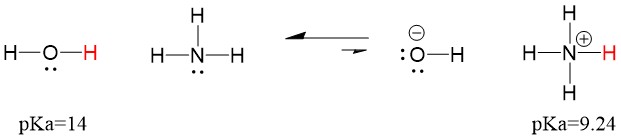
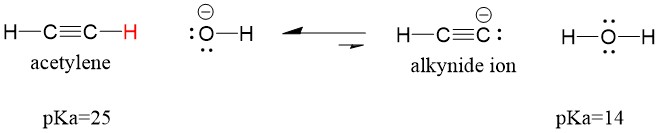
Many students find themselves struggling by the end of semester in Orgo 1. To make matters worse, you are now staring at a difficult class in Orgo 2 next semester. How can you possibly get ready for this difficult class? We at StudyOrgo are here to say, you CAN make it! But to do it efficiently, you will need the right tools to succeed. With winter break approaching, take advantage of this time to get ahead NOW, and with StudyOrgo you can really accelerate your success next semester.
Patch up your understanding with StudyOrgo
The best way to stay ahead for next semester is to divide your chapters into blocks that you will study. Most professors will have a fairly clear outline of what chapters from your book will be covered before each exam. Your goal here should be to divide your time (starting right now!) until the exam into blocks to study. Some chapters, like substitution (e.g. SN2) and elimination (e.g. E2), are foundations for future reactions you will learn later in Orgo 2. So, spend a lot of time revisiting these important topics if you felt lost in Orgo 1. Finished a chapter early? Many people find studying before the lecture help them to “feel” they really understand what the professor is saying and gives you an opportunity to ask questions right away. Don’t have a book, or your book is terrible??? Check out the over 180 reactions at StudyOrgo that are clearly organized and expained!
Schedule your studying times
To force yourself to get the studying done, get serious about it! The best way to do this is to schedule your studying as an “appointment.” Carry out your studying at a designated spot, we recommend not studying in the comfort of your home or dorm room where distractions are everywhere. Choose a coffee shop, library or classroom to force yourself into studying. Learning these time management skills will not only help you earn a passing grade in Orgo 2, it will help you in your career as well!
Read one chapters of the book per day, and just read it.
When its winter break, it can be hard to get motivated. If its the end of the semester, there doesnt feel like there is enough time! In either case, we strongly suggest starting by just reading the chapter. Don’t stop to define words or look up something, just read it! Put it down for a day and then read it again tomorrow. This time, try to define terms you don’t understand by using our powerful platform of topics to help clarify common conceptual problems. Then read it a third time, you should be feeling familiar with the topics now, and you are ready to start putting it to use in the practice problem set! Can you draw the mechanism? Can you identify the correct reagents? Here are StudyOrgo, we present all of our mechanisms. Use our flashcard method for Review Mode for learning or Quiz Mode to help drill yourself on the problems.
Practice Problems
Professors famously tell their students homework is not mandatory and you will not be graded on the homework. Relax then, right? Wrong! Its important to understand that there are only so many types of questions a professor can ask. So, if you see a hundreds, and we mean hundreds, of practice problems, then chances are you have already seen the type of problems that will be on the test. Many professors will throw in “really hard” questions that terrify students and it may seem like they are being unfair. There is a reason for everything! Professors use this strategy to assign A’s to the students who have kept up and followed along the whole time. And rightfully so since these questions cannot be answered without understanding everything they have covered. You can be one of the few who aces these questions! We have comprehensive reviews of the mechanism, stereoselectivity, regioselectivity, reaction considerations and more!
Review Materials before the Exam
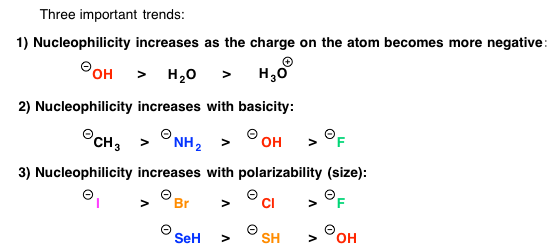
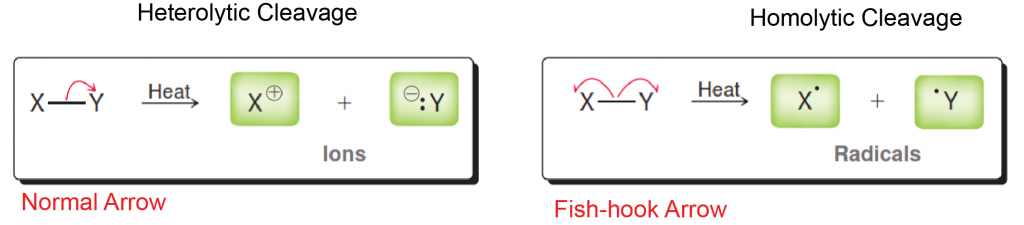
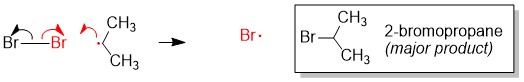
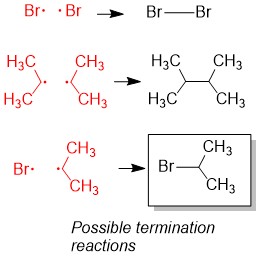
Either you have been following the steps above the whole semester and you are ready to prepare for Orgo 2 this winter break… or you read our article too late and the exam is next week. There is still hope! Simply adjust your strategy to fit the time you have remaining until the exam. We suggest you review ALL of the material though and not just what you think you don’t know. In our combined 30 years of experience!!!, the difficulties students have are often because they missed a concept earlier on. Do you know all of these topics?? If not, use StudyOrgo to patch up your understanding, or to simply check yourself. We’ll bet you’ll find something you didn’t know in one of these topics!
Remember, Organic Chemistry is like a block in the pyramid to get to your science or medical career goal. The top falls without a strong base.
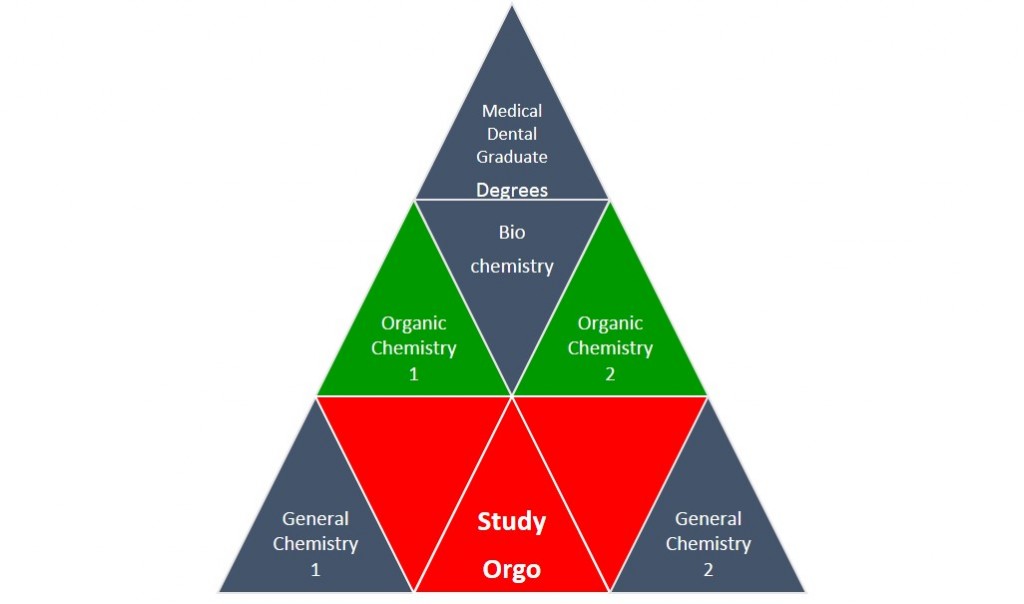
Here at StudyOrgo, we have developed easy to follow review study guides and exercise sets to help with reviewing all the concepts you will have to master to pass the course! Check out www.studyorgo.com/summary.php for help with core topics in Orgo 1 that you will need to succeed for next semester!