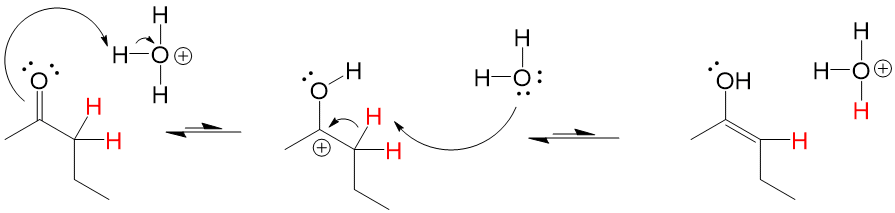
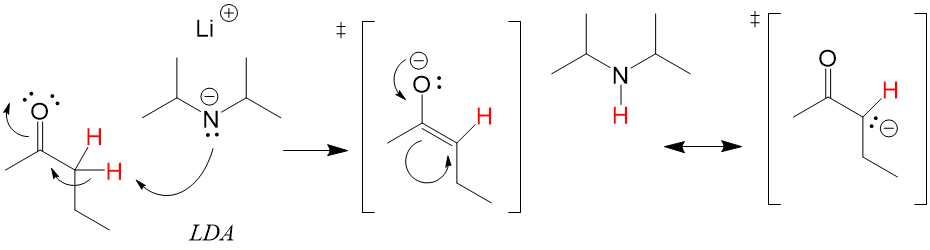
In this post, we will discuss the concepts and theories of Thermodynamics. While the topic sounds complicated, we at StudyOrgo have extensive experience instructing principles and reaction mechanisms frequently covered in Organic Chemistry. Sign up today for clear, detailed explanations of over 180 Orgo Chem reactions and reviews on conceptual topics!
The Equilibrium Constant
Remember from general chemistry that equilibrium constant (Keq) reflect the ratio of products to reactants in the following equation.
Keq = Products / Reactants = [C]*[D] / [A] * [B]
Thus, if one knows the concentration of product or reactant in solution and the Keq for the reaction, the other value can be also know. One point of confusion is that these values should somehow be intuitively known. They are calculated in the lab for each reaction, with each type of reactants. Therefore, without the “empirical” data we wouldn’t know the Keq. Fortunately, many experiments have been done in the world and Keq values are frequently found in tables and reference materials.
Spontaneity and Gibbs Free Energy
An important factor in choosing reactions in organic chemistry is knowing how “efficient” this reaction will be. Since our goal to generate products, knowing the Keq for the reaction will allow us to predict how much product we can theoretically produce from a known starting concentration of reactants. We can convert the Keq ratio into energy (kJ/mole) which is a more comparable measurement between reactions using a version of the Gibbs Free Energy equation.
dG = RT*ln[Keq]
Remember, that a release of energy (a negative dG) indicates a spontaneous and forward (towards product) reaction. As a reference, let’s look at a table of corresponding dG and Keq values and how this relates to concentration of products.
| Delta G (kj/mol) | Keq value | % products at equilibrium |
| +17 | 1×10^3 | 99.90% |
| +11 | 1×10^2 | 99% |
| +6 | 1×10^1 | 90% |
| 0 | 1 | 50% |
| -6 | 1×10^-1 | 10% |
| -11 | 1×10^-2 | 1% |
| -17 | 1×10^-3 | 0.10% |
When dG is negative (exothermic), the Keq is very high (products >> reactants) and we see that most values have a >90% concentration of products when the reaction reaches equilibrium. However, when dG is positive (endothermic), the Keq is very low (products << reactants) and we see most values have <10% products.
Therefore, when selecting reactions in your synthesis scheme you will likely want to choose reactions with the highest Keq and most exothermic to generate high concentration.
The reaction coordinate
Reaction coordinates are often used to visualize concepts of free energy between the reactants and products of reactions. Below are examples of endothermic and exothermic reactions.
The Y-axis reflects the free energy of the reactants (A + B) and products (C + D). The X-axis reflects the progress of the reaction, as the path between reactants and products will have several hills and valleys that reflect transition states and intermediates, respectively which we will cover in another article. The thermodynamics of a reaction can all be visualized from graphs like these. You will see them a lot in the course!
Determining Gibbs Free Energy from reaction Coordinate
The difference between initial reactant (blue) and final product (green) free energies will give you the Gibbs Free Energy (dG) of the reaction. Remember, negative = spontaneous and positive = not spontaneous. The larger the value, the more complete the reaction goes to either side. For example, a large positive value means low products (no reaction) and large negative value means high products (efficient reaction).
Here at StudyOrgo, we have developed easy to follow review study guides and exercise sets to help with reviewing all the concepts you will have to master to pass the course! Check out www.studyorgo.com/summary.php for help with core topics in Orgo 1 that you will need to succeed for next semester!