Part Ten: Introduction to the Study of Orgo Reactions
- Reaction Format
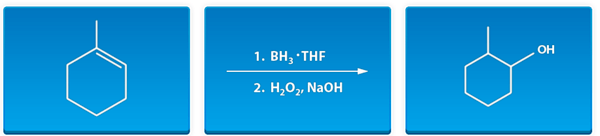
- Organic chemistry reactions are typically presented in the following format:
- Reactants or reagents are mixed together to form product(s).
- Reactants and reagents are placed to the left and on top of an arrow which point to the product(s).
- If certain reagents are added separately from one another in a particular sequence, then numbers are used to indicate the order in which they are added.
- Example:
- Reaction Mechanism and Intermediates
- Most reactions occur over a span of multiple steps. This is commonly known as the reaction mechanism. The mechanism is written in a step-wise fashion using arrows to demonstrate where the electrons are going from step to step.
- Each compound created along the way is known as a reaction intermediate.
- Degree of Substitution in Alkanes
- Carbon atoms in alkanes may be bonded to up to four other carbon atoms. We use the terms primary, secondary, and tertiary, quaternary to refer to the degree of substitution level that a given carbon has in a molecule.
- This is important because the fate of how certain reactions proceed depends on these substitutions levels
- To figure out the substitution level of any given carbon, follow these three easy steps:
- Step #1: Pick a carbon
- Step #2: Count how many carbons are directly attached to it. Other elements such as hydrogen, nitrogen, oxygen etc. don't count.
- Step #3: Give it a label:
- Primary= a carbon attached to only one other carbon
- Secondary= a carbon attached to only two other carbon
- Tertiary = a carbon attached to only three other carbon
- Quaternary = a carbon attached to only four other carbon
- In the following example, the carbons are color-coded to highlight their degree of substitution:
- Primary
- Secondary
- Tertiary
- Bear in mind that hydrogens attached to a given carbon also take on the labels as described in step #3 above:
- Primary = a hydrogen on a carbon attached to only one other carbon
- Secondary = a hydrogen on a carbon attached to only two other carbons
- Tertiary = a hydrogen on a carbon attached to three other carbons
- Degree of Substitution in Alkenes
- Carbon atoms on either side of a double bond may each bond up to two other carbons
- The more carbons attached to the double bond, the more highly substituted the alkene is
- This is important because the fate of how certain reactions proceed depends on these substitutions levels. Also a certain degree of substitution may be favored in certain reaction products.
- Allylic position
- Definition: The position immediately next to a double bond
- This is important because this position is typically favored by certain reactive intermediates
- Reaction Intermediates
- Radical
- Typically electrons come in pairs. However there are unpaired electrons known as radical electrons. These are usually just called radicals.
- Radical stability
- Radicals prefer a greater degree of alkyl substitution. Even more so, radicals prefer to be in the allylic position. Therefore here is the hierarchy of radical intermediate stability:
- Carbocation
- Carbocations serve as electrophiles in reactions. They will attract electrons easily as the carbon is deficient in electrons.
- Carbocation stability
- Carbocations prefer a greater degree of alkyl substitution. Even more so, carbocations prefer to be in the allylic position. Therefore here is the hierarchy of carbocation intermediate stability:
- Carbanion
- Carbanions serve as nucleophiles in reactions. They will donate electrons easily as the carbon has excess electrons.
- Carbanion stability
- Carbanions prefer a lesser degree of alkyl substitution. Even more so, carbanions prefer to be in the allylic position. Therefore here is the hierarchy of carbanion intermediate stability: