Part Eight: Isomers
- Isomers Overview
Isomers = compounds that are not the same but share the same molecular formula
Isomer Type Example Structural Isomers = constitutional isomers Compounds that have a difference in the order inwhichthe components are bonded
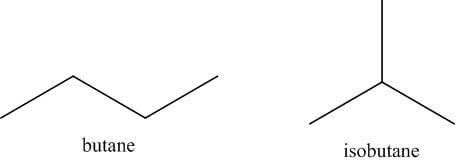
Conformational Isomers Compounds that differ in the rotation about a single bond
Stereoisomers Compounds that differ in the way they are oriented
A Diastereomers Stereoisomers that are NOT mirroimages
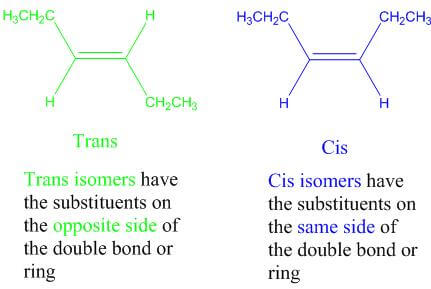
A-1 Geometric Isomers Diastereomers that differ specifically regarding the orientation of the substituents of a double bond or ring in a cis or trans orientation
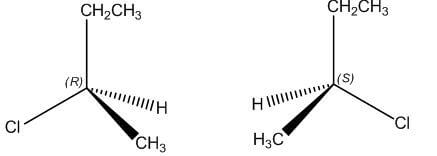
B Enantiomers Stereoisomers that are mirror images
- Conformational Isomers Analysis
- Newman projections = a way to depict the different arrangements formed by rotation about the single bond of a molecule
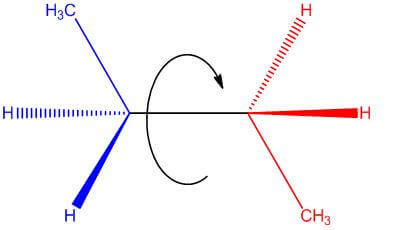
- For example, in this conventional drawing of butane, one can appreciate that the structure can be rotated about its single bond
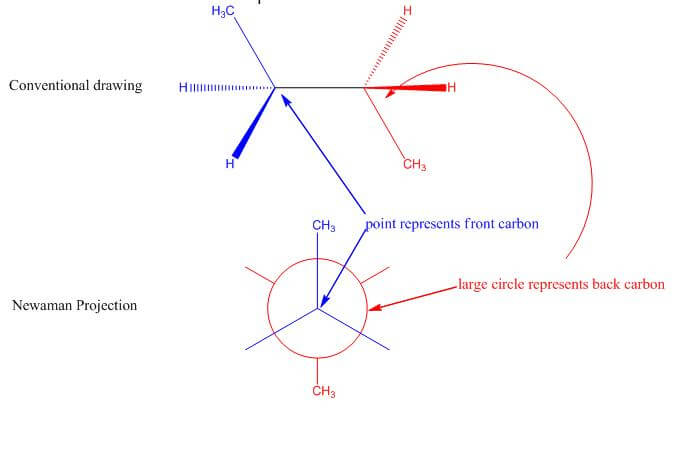
- Understanding Newman projections:
The following images of the traditional structure drawing and corresponding Newman projection are color coordinated to demonstrate their relationship:
- For example, in this conventional drawing of butane, one can appreciate that the structure can be rotated about its single bond
- What are the different types of conformational isomers? Here we define them by using butane as an example. We will go through the conformers by starting in the totally eclipsed position and rotate about the single bond from there.
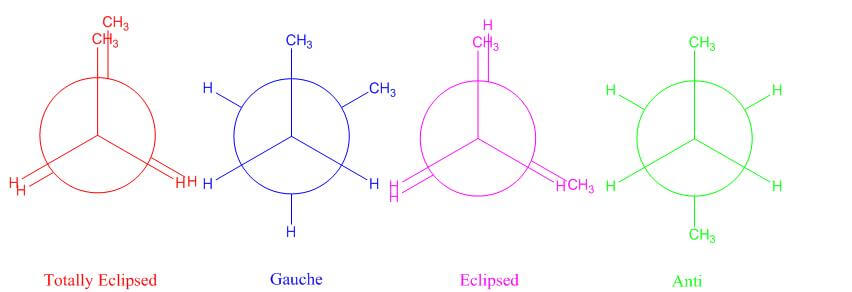
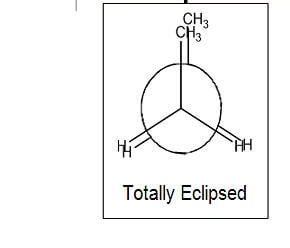
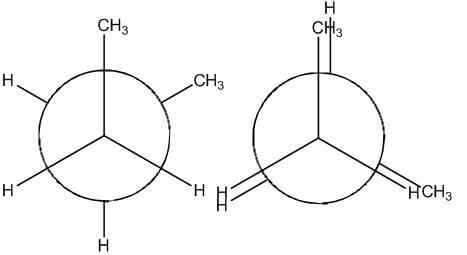
- Totally Eclipsed - methyl groups are aligned with one another
- There is torsional strain between groups
- Repulsion of electron clouds
- There is steric hindrance
- The interference between two bulky groups
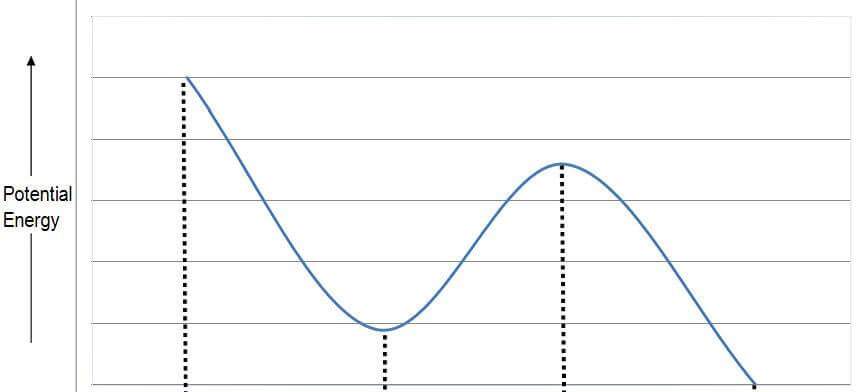
- These are the least stable of the conformations
- They possess the highest potential energy of the conformations
- There is torsional strain between groups
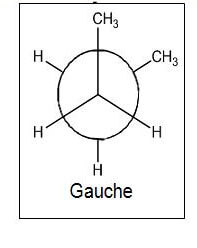
- Gauche- When the methyl groups are staggered to the left or right of one another at a 60° angle
- More stable
- Lower potential energy
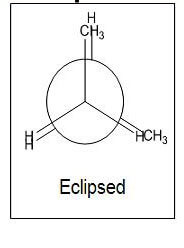
- Eclipsed- Eclipsed position, but methyl groups are not eclipsed and are at a 120° angle to one another
- Less stable
- Higher potential energy
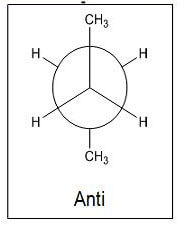
- Anti- When the methyl groups are staggered to be completely opposite of one another and are at a 180° angle
- Most stable
- Least potential energy
- Totally Eclipsed - methyl groups are aligned with one another
- Chair conformations
- Cyclohexane can be drawn in its conventional form, or in a variety of 3-D like model drawings. An important such conformation is known as a chair conformation.
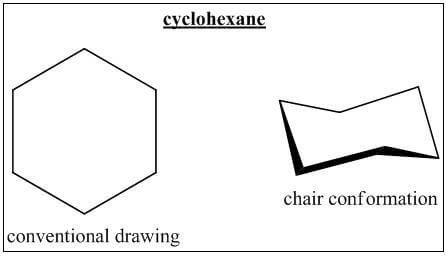
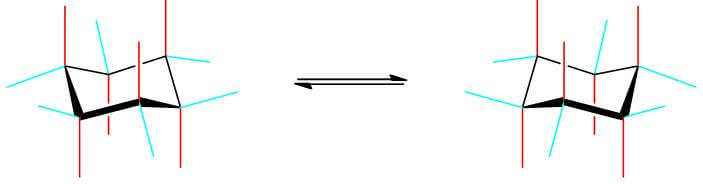
- The chair conformation can flip between two different positions:

- Positions on the chair conformation
- Equatorial: Position nearly parallel to the overall axis of the structure.
- Axial: Position nearly perpendicular to the overall axis of the structure.
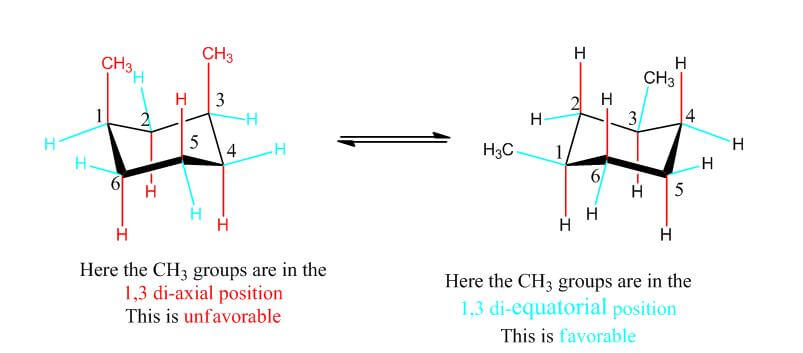
- The chair conformations demonstrate the importance of minimizing repulsion between groups to increase stability of a molecule
- 1,3 di-axial versus 1,3 di-equatorial
- 1,3 di-axial= very unfavorable due to steric hindrance between bulky groups
- 1,3 di-equatorial = much more favorable as steric hindrance is minimized and the structure is much more stable
- Cyclohexane can be drawn in its conventional form, or in a variety of 3-D like model drawings. An important such conformation is known as a chair conformation.
- Newman projections = a way to depict the different arrangements formed by rotation about the single bond of a molecule
Part Eight Exercise Set
Instructions:
- Use what you learned in Part Eight to complete the following exercise.
- Below is a potential energy diagram for conformational isomers of butane.
- Each point on the x-axis corresponds to a different conformational isomer.
- Choose from the following images to place at the appropriate point on the x- axis below:
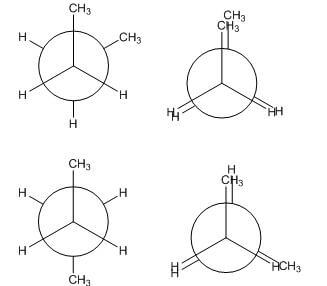
- When ready click on click on any window to reveal the correct conformer.